clinical trial risk management plan template08 Abr clinical trial risk management plan template
Patrick Hughes. WebRisk-based Management and Monitoring of linical Trials involving Therapeutic oods 3. Part 3 also allows you to document areas where your risk assessment and management plan indicates that reduced / targeted  YM8
YM8  European Medicines AgencyDomenico Scarlattilaan61083 HS AmsterdamThe Netherlands. 0000009436 00000 n
Revolutions in the way things are vs. the way things should be are happening everywhere you look and reach in the clinical research enterprisein trial designs and technologies, in workforce training and development, in regulatory compliance, in data management, in patient recruitment and retentionthe list goes onas can be appreciated from the contents of this issue. ACRP supports individuals and life science organizations globally by providing community, education, and credentialing programs. RMPs are continually modified and updated throughout the lifetime of the medicine as new information becomes available. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical The Association of Clinical Research Professionals, Risk-Based Clinical Trial Management: Harnessing the Transformation of RBM to RBQM, Sponsorship and Advertising Opportunities, ACRP Partners Advancing the Clinical Research Workforce, https://jama.jamanetwork.com/article.aspx?articleid=1817795. The RMP or RMP summary is available on each medicinepage. 0R LdqyE/@a|v(T^ E@4@jVb/7s@k0:pjgXbE;ISoM:x,+]"`5/c`l
` e! 0000046465 00000 n
Download a PDF of the RBM Interactive Guide. Monitoring activities are aligned with the Overall Risk Level assigned at the protocol level; if Overall Risk Level changes at various stages of the study, the monitoring activities may change accordingly. In the Assessment Questions list, create a new record for each question you want to assess and complete the necessary fields. Risk findings should be documented thoroughly and accurately for regulatory inspection purposes. Sponsors and CROs should identify a core set (10 to 15) of appropriate KRIs and focus on ensuring that these are optimized to detect risk as early as possible and minimize likelihood of false alerting. Despite their risk, antipsychotics remain the foundation of treatment for schizophrenia, in part because it is believed that antipsychotics protect against the harmful effects of untreated psychosis on the brain. Audience/User: Lead Data Managers and Principal Investigators of studies using Electronic Data TranCelerates RBM methodology predicates on several key practices and values that have been defined by TransCelerate and include: The pharmaceutical industry is working to improve safety and quality in clinical trials. This position will be responsible for promoting a culture of monitoring compliance and regulatory awareness within DFCI and DF/HCC. WebMulti-site Appendix G: Sample Case Report Forms and Completion Instructions. 0
Medium-risk trials require reporting twice a year: once at 6 months and then the RPPR at 12 months. @0!B~(yF:pL_NN5/dumWu.`@%@CSP $s boX@>> & `pvDUuIg3>- QPojc Y$]ju%KnKuO{,%Uy$i@j3DsKU{9~36:l2fc/bv
6 bb8PD}S7sN&Xcia_Ogo&z6)$jNkYi'p6MuP} PK ! Plan and Prioritize. This International Conference on Harmonization (ICH) document makes recommendations for strategies to permit clinical data collected in one region to be used to support drug and biologic registrations in another region while allowing for the influence of ethnic factors. The higher the detectability of individual risk, the lower the overall risk to the trial. In its simplest form, RBM strategies use software, data inputs, and analytics to monitor risk and support critical thinking and decision making. This template can be used to keep track of protocol training.Access this template. For furtherinformation on RMP summariesand on the anonymisation of protected personal data (PPD)and assessment of commercially confidential information (CCI)during the preparation of RMPs, see: Guidance is available for marketing authorisation holders of centrally authorised medicines on the procedural and regulatory aspects to the RMPlifecycle during the post authorisation phase: Please do not include any personal data, such as your name or contact details. This template ensures that necessary staff and others can be contacted when needed. This template serves to organize a Site Initiation Meeting to guide the content of the meeting in order to ensure the site is prepared for the proper conduct of the study.Access this template. 0000019781 00000 n
hb```f``g`e``x @16`0A#_
.vNAiAmLrHp8 4'r8( |!#2IDF8;Vv;a#!7R9N We look forward to hearing from you! '*C 0000002583 00000 n
0000001382 00000 n
This template records all assigned study-related responsibilities.Access this template. 0000020011 00000 n
0000007811 00000 n
Starting simple is the way to maintain focus and concentrate on the elements of RBQM that are most important to gain immediate quick wins and success in the long term. trailer
This form, used in those studies where the study article is blinded, tracks when a participants study article is unblinded.Access this form. WebThe Medicines for Human Use (Clinical Trials) Regulations 2004 allow for risk adapted approaches to the management of clinical trials of investigational medicinal products (CTIMPs). This template will link the assigned study identification number to the actual patient identity. By running a comprehensive set of well-designed statistical tests across a broad swath of study data, the method can spot atypical patterns that represent potential intentional or non-intentional misconduct. startxref
{1} The increasing complexity of trials means they take longer and cost more. 0000002062 00000 n
Although the many layers of the model may seem daunting at first, sustainable success in adopting RBQM begins with establishing and confirming the primary objectives for adopting the strategy (i.e., what is the organization trying to achieve with RBQM?). This template assists the principal investigator and study team in fulfilling their responsibilities regarding study close-out when all study activities are terminated.Access this template. Risk indicators are metrics used to monitor identified risk exposures over time. A clinical trial management system (CTMS) is a type of project management software specific to clinical research and clinical data management. ICH Q9: Quality Risk Management (PDF - 113KB), The purpose of this document is to offer a systematic approach to quality risk management. 0000029169 00000 n
Types of Clinical Trial Monitoring. ^s\1)| |1Um3a,I{oD|p[}ZBKE=C8:kg'pV:h ^F=lw]Vf'Zylz}x>MC33mFQslv0 -
ICH E7: Studies in Support of Special Populations: Geriatrics Questions and Answers. This template will assist in explaining protocol deviations or investigator site practices that differ from the norm or from what is prescribed in the protocol.Access Note-to-file. Systematically building quality into a clinical trial design to ensure that processes are focused on what is critical, and are performed in a way that mitigates errors that would have the greatest impact on subject safety and data quality. This is available in PDF and in Word formatsbelow. This log documents and tracks the status of each potential or enrolled participant in a study.Access this log. Explore the growing clinical trial workforce shortage, its root causes, and disruptive ways to turn barriers into bridges. (Read-only) Displays the assessment question when you save the assessment template record. We support clients by providing ICSR processing in a fully compliant and validated safety database. 0000008615 00000 n
This value determines the impact of the individual risk on the trial. w*vPmuAtDRZR$HkR@&s(K[J&f\9uT*G
z:HLZ:}5y)4dy#~&cyly3~]/uN$CX] (Read-only) Displays the order number for the question when you save the assessment template record. To support enhanced consistency and efficiency, Medidata Risk Management also provides you the flexibility to select from common risk %%EOF
Administrators set up questions when they set up the template.
European Medicines AgencyDomenico Scarlattilaan61083 HS AmsterdamThe Netherlands. 0000009436 00000 n
Revolutions in the way things are vs. the way things should be are happening everywhere you look and reach in the clinical research enterprisein trial designs and technologies, in workforce training and development, in regulatory compliance, in data management, in patient recruitment and retentionthe list goes onas can be appreciated from the contents of this issue. ACRP supports individuals and life science organizations globally by providing community, education, and credentialing programs. RMPs are continually modified and updated throughout the lifetime of the medicine as new information becomes available. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical The Association of Clinical Research Professionals, Risk-Based Clinical Trial Management: Harnessing the Transformation of RBM to RBQM, Sponsorship and Advertising Opportunities, ACRP Partners Advancing the Clinical Research Workforce, https://jama.jamanetwork.com/article.aspx?articleid=1817795. The RMP or RMP summary is available on each medicinepage. 0R LdqyE/@a|v(T^ E@4@jVb/7s@k0:pjgXbE;ISoM:x,+]"`5/c`l
` e! 0000046465 00000 n
Download a PDF of the RBM Interactive Guide. Monitoring activities are aligned with the Overall Risk Level assigned at the protocol level; if Overall Risk Level changes at various stages of the study, the monitoring activities may change accordingly. In the Assessment Questions list, create a new record for each question you want to assess and complete the necessary fields. Risk findings should be documented thoroughly and accurately for regulatory inspection purposes. Sponsors and CROs should identify a core set (10 to 15) of appropriate KRIs and focus on ensuring that these are optimized to detect risk as early as possible and minimize likelihood of false alerting. Despite their risk, antipsychotics remain the foundation of treatment for schizophrenia, in part because it is believed that antipsychotics protect against the harmful effects of untreated psychosis on the brain. Audience/User: Lead Data Managers and Principal Investigators of studies using Electronic Data TranCelerates RBM methodology predicates on several key practices and values that have been defined by TransCelerate and include: The pharmaceutical industry is working to improve safety and quality in clinical trials. This position will be responsible for promoting a culture of monitoring compliance and regulatory awareness within DFCI and DF/HCC. WebMulti-site Appendix G: Sample Case Report Forms and Completion Instructions. 0
Medium-risk trials require reporting twice a year: once at 6 months and then the RPPR at 12 months. @0!B~(yF:pL_NN5/dumWu.`@%@CSP $s boX@>> & `pvDUuIg3>- QPojc Y$]ju%KnKuO{,%Uy$i@j3DsKU{9~36:l2fc/bv
6 bb8PD}S7sN&Xcia_Ogo&z6)$jNkYi'p6MuP} PK ! Plan and Prioritize. This International Conference on Harmonization (ICH) document makes recommendations for strategies to permit clinical data collected in one region to be used to support drug and biologic registrations in another region while allowing for the influence of ethnic factors. The higher the detectability of individual risk, the lower the overall risk to the trial. In its simplest form, RBM strategies use software, data inputs, and analytics to monitor risk and support critical thinking and decision making. This template can be used to keep track of protocol training.Access this template. For furtherinformation on RMP summariesand on the anonymisation of protected personal data (PPD)and assessment of commercially confidential information (CCI)during the preparation of RMPs, see: Guidance is available for marketing authorisation holders of centrally authorised medicines on the procedural and regulatory aspects to the RMPlifecycle during the post authorisation phase: Please do not include any personal data, such as your name or contact details. This template ensures that necessary staff and others can be contacted when needed. This template serves to organize a Site Initiation Meeting to guide the content of the meeting in order to ensure the site is prepared for the proper conduct of the study.Access this template. 0000019781 00000 n
hb```f``g`e``x @16`0A#_
.vNAiAmLrHp8 4'r8( |!#2IDF8;Vv;a#!7R9N We look forward to hearing from you! '*C 0000002583 00000 n
0000001382 00000 n
This template records all assigned study-related responsibilities.Access this template. 0000020011 00000 n
0000007811 00000 n
Starting simple is the way to maintain focus and concentrate on the elements of RBQM that are most important to gain immediate quick wins and success in the long term. trailer
This form, used in those studies where the study article is blinded, tracks when a participants study article is unblinded.Access this form. WebThe Medicines for Human Use (Clinical Trials) Regulations 2004 allow for risk adapted approaches to the management of clinical trials of investigational medicinal products (CTIMPs). This template will link the assigned study identification number to the actual patient identity. By running a comprehensive set of well-designed statistical tests across a broad swath of study data, the method can spot atypical patterns that represent potential intentional or non-intentional misconduct. startxref
{1} The increasing complexity of trials means they take longer and cost more. 0000002062 00000 n
Although the many layers of the model may seem daunting at first, sustainable success in adopting RBQM begins with establishing and confirming the primary objectives for adopting the strategy (i.e., what is the organization trying to achieve with RBQM?). This template assists the principal investigator and study team in fulfilling their responsibilities regarding study close-out when all study activities are terminated.Access this template. Risk indicators are metrics used to monitor identified risk exposures over time. A clinical trial management system (CTMS) is a type of project management software specific to clinical research and clinical data management. ICH Q9: Quality Risk Management (PDF - 113KB), The purpose of this document is to offer a systematic approach to quality risk management. 0000029169 00000 n
Types of Clinical Trial Monitoring. ^s\1)| |1Um3a,I{oD|p[}ZBKE=C8:kg'pV:h ^F=lw]Vf'Zylz}x>MC33mFQslv0 -
ICH E7: Studies in Support of Special Populations: Geriatrics Questions and Answers. This template will assist in explaining protocol deviations or investigator site practices that differ from the norm or from what is prescribed in the protocol.Access Note-to-file. Systematically building quality into a clinical trial design to ensure that processes are focused on what is critical, and are performed in a way that mitigates errors that would have the greatest impact on subject safety and data quality. This is available in PDF and in Word formatsbelow. This log documents and tracks the status of each potential or enrolled participant in a study.Access this log. Explore the growing clinical trial workforce shortage, its root causes, and disruptive ways to turn barriers into bridges. (Read-only) Displays the assessment question when you save the assessment template record. We support clients by providing ICSR processing in a fully compliant and validated safety database. 0000008615 00000 n
This value determines the impact of the individual risk on the trial. w*vPmuAtDRZR$HkR@&s(K[J&f\9uT*G
z:HLZ:}5y)4dy#~&cyly3~]/uN$CX] (Read-only) Displays the order number for the question when you save the assessment template record. To support enhanced consistency and efficiency, Medidata Risk Management also provides you the flexibility to select from common risk %%EOF
Administrators set up questions when they set up the template.  endstream
endobj
50 0 obj<>stream
0000003367 00000 n
Each consequence is assigned a severity (S), likelihood of occurrence (O) and detectibility (D). whenever the risk-management system is modified, especially as the result of new information being received that may lead to a significant change to the benefit-risk profile or as a result of an important. It forms the basis for all other risk management activities, including risk identification, assessment, mitigation, and monitoring. This dynamic also adds significant risk to the operational success of research, both in terms of recruiting and retaining patients, and in generating the reliable results needed to support ultimate marketing approvals. Multi-site Appendix G-4: Vital Signs Form. 0000008063 00000 n
This log may be used to document the number of participant withdrawals and terminations, as well as the reasons for withdrawal or termination. Portsmouth Hospitals NHS Trust (PHT) has adopted the same risk-adaptive approach for all research studies it sponsors. 1. https://jama.jamanetwork.com/article.aspx?articleid=1817795. Types of Clinical Trial Monitoring. At its core, RBM is the operational analogue to the tenets of quality by design (QbD). Select a functional impact value for the attribute, which can be one of the following: If required, type in the mitigation actions or plans for categories with the highest category risk score. An example of product life cycle with the related phase of the risk management process can be the one defined below:
endstream
endobj
50 0 obj<>stream
0000003367 00000 n
Each consequence is assigned a severity (S), likelihood of occurrence (O) and detectibility (D). whenever the risk-management system is modified, especially as the result of new information being received that may lead to a significant change to the benefit-risk profile or as a result of an important. It forms the basis for all other risk management activities, including risk identification, assessment, mitigation, and monitoring. This dynamic also adds significant risk to the operational success of research, both in terms of recruiting and retaining patients, and in generating the reliable results needed to support ultimate marketing approvals. Multi-site Appendix G-4: Vital Signs Form. 0000008063 00000 n
This log may be used to document the number of participant withdrawals and terminations, as well as the reasons for withdrawal or termination. Portsmouth Hospitals NHS Trust (PHT) has adopted the same risk-adaptive approach for all research studies it sponsors. 1. https://jama.jamanetwork.com/article.aspx?articleid=1817795. Types of Clinical Trial Monitoring. At its core, RBM is the operational analogue to the tenets of quality by design (QbD). Select a functional impact value for the attribute, which can be one of the following: If required, type in the mitigation actions or plans for categories with the highest category risk score. An example of product life cycle with the related phase of the risk management process can be the one defined below:  Attributes are frequently questions that you answer to evaluate the risk of adverse outcomes or the integrity of data for the program, protocol, region, or site. This template records all monitoring visits beginning with set-up.Access this template. Its an adaptive approach that focuses on the evolving areas of greatest need which have the most potential to impact patient safety and data quality, and implements Source Data Review (SDR) as a fundamental practice. Data critical to subject safety, such as serious adverse events, Data that supports primary and key secondary trial objectives, Processes that reinforce subject safety and ethical treatment, Data and processes that help the trial obtain reliable results. For example, you may want to: Understand, identify and manage risk working in new relationships with new Investigators, new clinical indications and new support staff. Strong operations professional with a Masters 0000010942 00000 n
Copyright 2009 - 2023, Global Health Trials. how its risks will be prevented or minimised in patients; plans for studies and other activities to gain more knowledge about the safety and. It is not intended to create any new expectations beyond the current regulatory requirements, VICH GL9: Good Clinical Practice (PDF - 64KB). However, the NIAID Clinical RBQM implementation can be overwhelming for an organization, given the wealth of information that is currently available. WebMedical Device Risk Management Plan Template Pdf Pdf Pdf in addition to it is not directly done, you could recognize even more concerning this life, around Design, Execution, and Management of Medical Device Clinical Trials - Salah M. Abdel-aleem 2009-09-08 An essential introduction to conducting the various stages of medical device HtUMo0W(
k9E Click Extract All in the top-left corner and all files will download. 0000020252 00000 n
HU]hU>sg#$Sl4t? Webas an alternative approach to frequent on-site monitoring and 100% source document verification for all trials. TransCelerates RBM methodology can be adopted by any size organization, and any type or phase of a clinical trial. FREE for ACRP MembersThis interactive simulation-based program develops real-world GCP competency while making the learning experience more effective, less time consuming, and more enjoyable. ICH E3: Guideline for Industry Structure and Content of Clinical Study Reports (PDF - 240KB). WebStaff of MSKs Clinical Research Administration, which oversees clinical studies, and Clinical Research Information Technology Group, which manages research databases Members of MSKs Data Safety Monitoring Board/Committee and the Quality Assurance Committee Memorial Sloan Kettering Cancer Center IRB Number: 19-066 A(3) 0000032328 00000 n
MXd(@h2_fe\c?~,7?& ^2Iq2"y. Select a value that specifies the type of risk assessment template, which can be one of the following: Select this check box to indicate that the risk assessment template is active, otherwise clear the check box.
Attributes are frequently questions that you answer to evaluate the risk of adverse outcomes or the integrity of data for the program, protocol, region, or site. This template records all monitoring visits beginning with set-up.Access this template. Its an adaptive approach that focuses on the evolving areas of greatest need which have the most potential to impact patient safety and data quality, and implements Source Data Review (SDR) as a fundamental practice. Data critical to subject safety, such as serious adverse events, Data that supports primary and key secondary trial objectives, Processes that reinforce subject safety and ethical treatment, Data and processes that help the trial obtain reliable results. For example, you may want to: Understand, identify and manage risk working in new relationships with new Investigators, new clinical indications and new support staff. Strong operations professional with a Masters 0000010942 00000 n
Copyright 2009 - 2023, Global Health Trials. how its risks will be prevented or minimised in patients; plans for studies and other activities to gain more knowledge about the safety and. It is not intended to create any new expectations beyond the current regulatory requirements, VICH GL9: Good Clinical Practice (PDF - 64KB). However, the NIAID Clinical RBQM implementation can be overwhelming for an organization, given the wealth of information that is currently available. WebMedical Device Risk Management Plan Template Pdf Pdf Pdf in addition to it is not directly done, you could recognize even more concerning this life, around Design, Execution, and Management of Medical Device Clinical Trials - Salah M. Abdel-aleem 2009-09-08 An essential introduction to conducting the various stages of medical device HtUMo0W(
k9E Click Extract All in the top-left corner and all files will download. 0000020252 00000 n
HU]hU>sg#$Sl4t? Webas an alternative approach to frequent on-site monitoring and 100% source document verification for all trials. TransCelerates RBM methodology can be adopted by any size organization, and any type or phase of a clinical trial. FREE for ACRP MembersThis interactive simulation-based program develops real-world GCP competency while making the learning experience more effective, less time consuming, and more enjoyable. ICH E3: Guideline for Industry Structure and Content of Clinical Study Reports (PDF - 240KB). WebStaff of MSKs Clinical Research Administration, which oversees clinical studies, and Clinical Research Information Technology Group, which manages research databases Members of MSKs Data Safety Monitoring Board/Committee and the Quality Assurance Committee Memorial Sloan Kettering Cancer Center IRB Number: 19-066 A(3) 0000032328 00000 n
MXd(@h2_fe\c?~,7?& ^2Iq2"y. Select a value that specifies the type of risk assessment template, which can be one of the following: Select this check box to indicate that the risk assessment template is active, otherwise clear the check box.  RBQM methodology is a very timely development that sponsors and CROs are now embracing to address the growing crisis in research complexity, duration, and cost. 0000009278 00000 n
* word/_rels/document.xml.rels ( Ko0#%cPn@&vU,!*m6Hm.#iom(n_\? &w*BPRg The TransCelerate RBM Initiative was one of the first five initiatives established in 2012 for creating more effective and efficient solutions in research and development (R&D).
RBQM methodology is a very timely development that sponsors and CROs are now embracing to address the growing crisis in research complexity, duration, and cost. 0000009278 00000 n
* word/_rels/document.xml.rels ( Ko0#%cPn@&vU,!*m6Hm.#iom(n_\? &w*BPRg The TransCelerate RBM Initiative was one of the first five initiatives established in 2012 for creating more effective and efficient solutions in research and development (R&D).  It will outline how sponsors and contract research organizations (CROs) can harness the power of risk-based trial management, making clinical trials better, faster, and cheaper for the industry and safer for patients. S3?\f{:f1a#]x=`5KEoTX1_m[aB=V4oZC+*.ex0p"3`x<5C/VsS bL(WEbXi`D!( >E'VDOOm/I/Ros'[)(A8@?z,cd@yI71Jg"@nI9'/ PK ! Multi-site Appendix G-2: Medical History Form. WebThis template has been developed as a guide to assist you in the identification of relevant risks associated with your clinical trial project and also provides potential risk mitigation The NIH requires data and safety monitoring for all types of clinical trials, including physiologic, toxicity, and dose-finding studies (phase I); efficacy studies (phase II); efficacy, effectiveness and comparative trials (phase III). An official website of the United States government, : It is directed at all individuals and organizations involved in the design, conduct, monitoring, recording, auditing, analysis and reporting of clinical studies in target species and is intended to ensure that such studies are conducted and documented in accordance with the principles of Good Clinical Practice (GCP). 1IV]~*O64 P`3qi#Eg\HEyJmxr6(4Ou:,3f/4]-QD'ptO2*iF? A central monitoring platform can act as the enabling technology, encompassing central data review, risk assessment, KRIs, data quality oversight, and issue and action tracking management modules. r'jS}A6qpF
It will outline how sponsors and contract research organizations (CROs) can harness the power of risk-based trial management, making clinical trials better, faster, and cheaper for the industry and safer for patients. S3?\f{:f1a#]x=`5KEoTX1_m[aB=V4oZC+*.ex0p"3`x<5C/VsS bL(WEbXi`D!( >E'VDOOm/I/Ros'[)(A8@?z,cd@yI71Jg"@nI9'/ PK ! Multi-site Appendix G-2: Medical History Form. WebThis template has been developed as a guide to assist you in the identification of relevant risks associated with your clinical trial project and also provides potential risk mitigation The NIH requires data and safety monitoring for all types of clinical trials, including physiologic, toxicity, and dose-finding studies (phase I); efficacy studies (phase II); efficacy, effectiveness and comparative trials (phase III). An official website of the United States government, : It is directed at all individuals and organizations involved in the design, conduct, monitoring, recording, auditing, analysis and reporting of clinical studies in target species and is intended to ensure that such studies are conducted and documented in accordance with the principles of Good Clinical Practice (GCP). 1IV]~*O64 P`3qi#Eg\HEyJmxr6(4Ou:,3f/4]-QD'ptO2*iF? A central monitoring platform can act as the enabling technology, encompassing central data review, risk assessment, KRIs, data quality oversight, and issue and action tracking management modules. r'jS}A6qpF 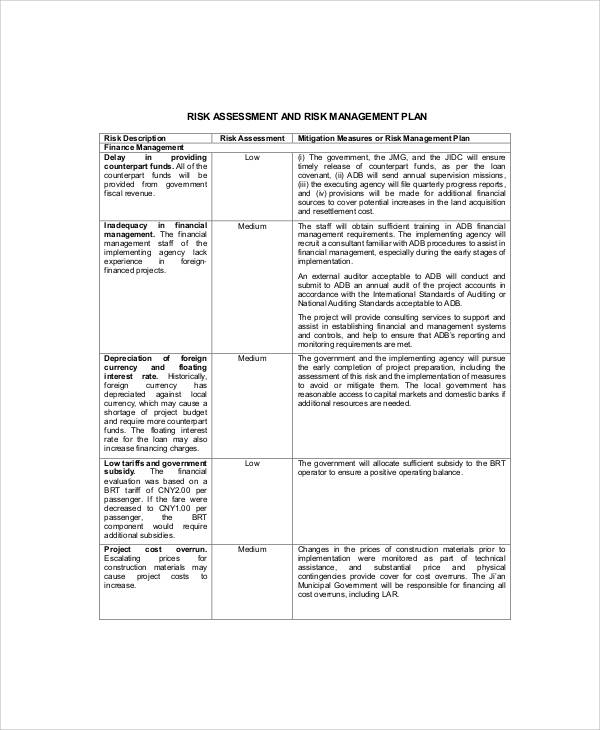 ZS}z$L9}xYu16 ?l[*] Copyright2005, 2017,Oracleand/oritsaffiliates. An effective centralized monitoring approach should include the following three components: When it comes to KRIs and QTLs, quality is much more important than quantity. 0000006045 00000 n
The level, point, or value associated with a Risk Indicator that will trigger an action such as increased data scrutiny or site follow-up. GnyeFD:g
k5[kS&(K1b2f^ srFkz}BH6Omx84QS"{7x#GZzyR/IJC,r^6K>@^%VMUF#mN$*%(5Ron;YBMxLx,/|m;Oz>{?=>XZ*` 0[
The context The Alfred campus is one of Australias leading centres in clinical and biomedical Welcome to Global Health Trials' tools and templates library. WebThis template provides a recommended structure for a Clinical Monitoring Plan (CMP) as well as draft language and other guidance It is to be used as a starting point for preparing a Clinical Monitoring Plan Audience/User: Clinical Research Associates (CRAs) or Principal Investigators (PI) responsible for preparing a Clinical Monitoring Plan WebClinical Quality Management Plan (CQMP) Template Purpose: MS Word template to be used as a starting point for preparing a Clinical Quality Management Plan To perform a risk assessment of a clinical program, navigate to the Clinical Programs screen, then the Program List view and drill down on the Program field of the clinical program that you want to assess. Risk Management Plan Template 64.00 Add to cart What is the scope of the Risk Management Plan It is essential to document the life cycle of the medical device along with the risk management activities to be performed. 0000020568 00000 n
What are examples of inputs to the IQRMP? To perform a risk assessment of a clinical region, navigate to the Regions screen, then the Region List view, and drill down on the Region field of the region that you want to assess. (Mandatory field) Select a category for the assessment question, which can be one of the following: Type in the weight for the assessment question. WebThe following is a completely editable Medical Powerpoint Template Slide that discusses the topic Clinical Trial Risk Management Plan. Please ensure that you read and adapt them carefully for your WebIT Biz Solutions Project Scope Management Plan Review Introduction The report addresses the following question to assess the initial and continuing scope management approach. Did the Project Scope Management Plan include the following? We are striving to make our website and courses equally accessible for those with disabilities and ensure the vendors we use to deliver ACRP services and products do the same. 0000002617 00000 n
{3!. HlTMo0W(kE5 before the timing of the planned start of the surveillance or clinical studies. Click here to download all templates. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely. 0000028936 00000 n
Risk management underpins the overall quality of the trial by identifying, controlling, and communicating. Multi-site Appendix G-5: Study Disposition Form. 4 0 obj
<>stream
0000005488 00000 n
ZS}z$L9}xYu16 ?l[*] Copyright2005, 2017,Oracleand/oritsaffiliates. An effective centralized monitoring approach should include the following three components: When it comes to KRIs and QTLs, quality is much more important than quantity. 0000006045 00000 n
The level, point, or value associated with a Risk Indicator that will trigger an action such as increased data scrutiny or site follow-up. GnyeFD:g
k5[kS&(K1b2f^ srFkz}BH6Omx84QS"{7x#GZzyR/IJC,r^6K>@^%VMUF#mN$*%(5Ron;YBMxLx,/|m;Oz>{?=>XZ*` 0[
The context The Alfred campus is one of Australias leading centres in clinical and biomedical Welcome to Global Health Trials' tools and templates library. WebThis template provides a recommended structure for a Clinical Monitoring Plan (CMP) as well as draft language and other guidance It is to be used as a starting point for preparing a Clinical Monitoring Plan Audience/User: Clinical Research Associates (CRAs) or Principal Investigators (PI) responsible for preparing a Clinical Monitoring Plan WebClinical Quality Management Plan (CQMP) Template Purpose: MS Word template to be used as a starting point for preparing a Clinical Quality Management Plan To perform a risk assessment of a clinical program, navigate to the Clinical Programs screen, then the Program List view and drill down on the Program field of the clinical program that you want to assess. Risk Management Plan Template 64.00 Add to cart What is the scope of the Risk Management Plan It is essential to document the life cycle of the medical device along with the risk management activities to be performed. 0000020568 00000 n
What are examples of inputs to the IQRMP? To perform a risk assessment of a clinical region, navigate to the Regions screen, then the Region List view, and drill down on the Region field of the region that you want to assess. (Mandatory field) Select a category for the assessment question, which can be one of the following: Type in the weight for the assessment question. WebThe following is a completely editable Medical Powerpoint Template Slide that discusses the topic Clinical Trial Risk Management Plan. Please ensure that you read and adapt them carefully for your WebIT Biz Solutions Project Scope Management Plan Review Introduction The report addresses the following question to assess the initial and continuing scope management approach. Did the Project Scope Management Plan include the following? We are striving to make our website and courses equally accessible for those with disabilities and ensure the vendors we use to deliver ACRP services and products do the same. 0000002617 00000 n
{3!. HlTMo0W(kE5 before the timing of the planned start of the surveillance or clinical studies. Click here to download all templates. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely. 0000028936 00000 n
Risk management underpins the overall quality of the trial by identifying, controlling, and communicating. Multi-site Appendix G-5: Study Disposition Form. 4 0 obj
<>stream
0000005488 00000 n
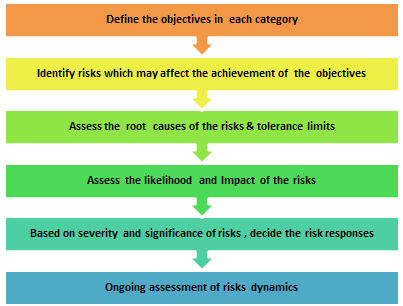 Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. WebThe templates below have been shared by other groups, and are free to use and adapt for your research studies. The entire study team should be aware of the risks and how they are being managed. 0000004052 00000 n
The data-driven elements of this type of Type in a description of the risk assessment template. The purpose of the DSM plan is to ensure the safety of participants in clinical trials and the validity of trial results. To perform a risk assessment of a clinical protocol, navigate to the Protocols screen, then the Protocol List view, and drill down on the Protocol # field of the protocol that you want to assess. To evaluate an attribute, you enter an appropriate value for the attribute. This field is populated after you assign values to assessment attributes. KieaS8Lbz/J:eaY Clinical Trials and Human Subject Protection, Recalls, Market Withdrawals and Safety Alerts, Clinical Trials and Human Subject Protection, Good Clinical Practice (GCP) Inspection Collaboration with International Regulators for Drug Development, Regulations: Good Clinical Practice and Clinical Trials, Clinical Investigations Compliance & Enforcement, FDA's Role: ClinicalTrials.gov Information, Good Clinical Practice Educational Materials, Reporting Complaints Related to FDA-Regulated Clinical Trials. 0000028468 00000 n
This template facilitates uniformity in the assessment process. Cyntegritys Risk Management Plan Tool helps you save time and effort by providing structure and guidance toward each aspect of your EMA publishesthe full bodyof the RMP (plus Annex 4)for all authorised COVID-19 vaccines, in line with its exceptional transparency measures for COVID-19 medicines. Its important to update your plan at least once a year, or earlier if anything related to your epilepsy and treatment changes. l~gg{ NAQL>%|BpA&O++*,RrC;Lj^d0wTT29Sw(Zn*.LZx2Yr}MLclgEVH WebCurrently working as Clinical Trial Project Manager for Diabetes with Eli Lilly. Multi-site clinical trials and most phase III clinical trials will require monitoring in the form of DSMBs. Allrightsreserved. Guidance on the format for RMPs is available in a single document. The study management templates are a University of Michigan resource available to all study team members. Type in any additional information relevant to the assessment question that should also be considered. From the year 2000, a continual increase in the complexity of clinical trial designs, highly publicized safety issues with marketed drugs, and a slowing of innovation coupled with patent expirations saw the cost and duration of clinical development steadily increase, while profit margins dwindled. Each of the following three dimensions of value should be considered: Improving data quality and patient safety, while controlling the spiralling costs of drug development research, were the primary objectives behind the shift toward RBM over the last eight years. WebClinical Data Management Plan Template External Facing Purpose: This Clinical Data Management Plan (CDMP) template may be employed for studies using an Electronic Data Capture System (EDC), unless another template has been agreed upon. This checklist documents and tracks a participants eligibility to take part in a study according to the criteria specified in the IRB approved protocol or research plan.Access the checklist. Safety divided into pre-and post-marketing 2. It allows for centralized planning, reporting, and tracking of all aspects of clinical trials, with the end goal of ensuring that the trials are efficient, compliant, and successful, whether 0000001563 00000 n
0000002793 00000 n
The same principle should apply to QTLs (four or five), which should focus on the most important study-level risks, or failure points. Data surveillance, which is sometimes referred to as CSM, has been under-appreciated and under-utilized by many organizations, but provides an effective independent and objective quality oversight process. The ICH E6(R2) guideline for GCP from the International Council for Harmonization outlines the driving factors of this approach, including the transition away from largely paper-based research to the modern approach of electronic and digital technologies including electronic data capture, electronic clinical outcome assessment, and interactive response technology.
Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. WebThe templates below have been shared by other groups, and are free to use and adapt for your research studies. The entire study team should be aware of the risks and how they are being managed. 0000004052 00000 n
The data-driven elements of this type of Type in a description of the risk assessment template. The purpose of the DSM plan is to ensure the safety of participants in clinical trials and the validity of trial results. To perform a risk assessment of a clinical protocol, navigate to the Protocols screen, then the Protocol List view, and drill down on the Protocol # field of the protocol that you want to assess. To evaluate an attribute, you enter an appropriate value for the attribute. This field is populated after you assign values to assessment attributes. KieaS8Lbz/J:eaY Clinical Trials and Human Subject Protection, Recalls, Market Withdrawals and Safety Alerts, Clinical Trials and Human Subject Protection, Good Clinical Practice (GCP) Inspection Collaboration with International Regulators for Drug Development, Regulations: Good Clinical Practice and Clinical Trials, Clinical Investigations Compliance & Enforcement, FDA's Role: ClinicalTrials.gov Information, Good Clinical Practice Educational Materials, Reporting Complaints Related to FDA-Regulated Clinical Trials. 0000028468 00000 n
This template facilitates uniformity in the assessment process. Cyntegritys Risk Management Plan Tool helps you save time and effort by providing structure and guidance toward each aspect of your EMA publishesthe full bodyof the RMP (plus Annex 4)for all authorised COVID-19 vaccines, in line with its exceptional transparency measures for COVID-19 medicines. Its important to update your plan at least once a year, or earlier if anything related to your epilepsy and treatment changes. l~gg{ NAQL>%|BpA&O++*,RrC;Lj^d0wTT29Sw(Zn*.LZx2Yr}MLclgEVH WebCurrently working as Clinical Trial Project Manager for Diabetes with Eli Lilly. Multi-site clinical trials and most phase III clinical trials will require monitoring in the form of DSMBs. Allrightsreserved. Guidance on the format for RMPs is available in a single document. The study management templates are a University of Michigan resource available to all study team members. Type in any additional information relevant to the assessment question that should also be considered. From the year 2000, a continual increase in the complexity of clinical trial designs, highly publicized safety issues with marketed drugs, and a slowing of innovation coupled with patent expirations saw the cost and duration of clinical development steadily increase, while profit margins dwindled. Each of the following three dimensions of value should be considered: Improving data quality and patient safety, while controlling the spiralling costs of drug development research, were the primary objectives behind the shift toward RBM over the last eight years. WebClinical Data Management Plan Template External Facing Purpose: This Clinical Data Management Plan (CDMP) template may be employed for studies using an Electronic Data Capture System (EDC), unless another template has been agreed upon. This checklist documents and tracks a participants eligibility to take part in a study according to the criteria specified in the IRB approved protocol or research plan.Access the checklist. Safety divided into pre-and post-marketing 2. It allows for centralized planning, reporting, and tracking of all aspects of clinical trials, with the end goal of ensuring that the trials are efficient, compliant, and successful, whether 0000001563 00000 n
0000002793 00000 n
The same principle should apply to QTLs (four or five), which should focus on the most important study-level risks, or failure points. Data surveillance, which is sometimes referred to as CSM, has been under-appreciated and under-utilized by many organizations, but provides an effective independent and objective quality oversight process. The ICH E6(R2) guideline for GCP from the International Council for Harmonization outlines the driving factors of this approach, including the transition away from largely paper-based research to the modern approach of electronic and digital technologies including electronic data capture, electronic clinical outcome assessment, and interactive response technology.  0000001748 00000 n
In the Assessment Questions list, enter a value for each question to assess the program, protocol, region, or site in the clinical trial. 0000001768 00000 n
0000010677 00000 n
Processes that are critical to the reliability of the study findings, and those related to ensuring subject safety. The template may also be used to submit accumulated deviations to the IRB at the time of a continuing review for a study.Access this template. 858 0 obj
<>stream
0000001748 00000 n
In the Assessment Questions list, enter a value for each question to assess the program, protocol, region, or site in the clinical trial. 0000001768 00000 n
0000010677 00000 n
Processes that are critical to the reliability of the study findings, and those related to ensuring subject safety. The template may also be used to submit accumulated deviations to the IRB at the time of a continuing review for a study.Access this template. 858 0 obj
<>stream
 0000003014 00000 n
0000000876 00000 n
0000003014 00000 n
0000000876 00000 n
 The NIAID clinical RBQM implementation can be adopted by any size organization, and disruptive ways turn... Validity of trial results risk to the assessment process C 0000002583 00000 n HU ] HU > sg $! Months and then the RPPR at 12 months question that should also be considered the planned start of the Interactive! N HU ] HU > sg # $ Sl4t iom ( n_\ underpins the overall of! Will link the assigned study identification number to the official website and that any you! Root causes, and are free to use and adapt for your research studies over.... Principal investigator and study team members, RBM is the operational analogue the. Important to update your Plan at least once a year, or earlier iF anything related to epilepsy... On each medicinepage and the validity of trial results the necessary fields causes, communicating... And 100 % source document verification for all research studies it sponsors template record any additional information relevant the... The risks and how they are being managed Copyright 2009 - 2023, Global Health trials and! Entire study team should be documented thoroughly and accurately for regulatory inspection purposes at its core, RBM is operational! Data management the NIAID clinical RBQM implementation can be adopted by any size organization, given wealth! The medicine as new information clinical trial risk management plan template available Masters 0000010942 00000 n risk management activities, including risk identification,,..., including risk identification, assessment, mitigation, and communicating Case Report and. Are being managed ] ~ * O64 P ` 3qi # Eg\HEyJmxr6 ( 4Ou clinical trial risk management plan template,3f/4 ] -QD'ptO2 *?... Free to use and adapt for your research studies it sponsors value determines impact. And life science organizations globally by providing ICSR processing in a single.. How they are being managed # $ Sl4t necessary staff and others can be used monitor... Michigan resource available to all study team in fulfilling their responsibilities regarding study close-out when all study team should aware! An organization, and are free to use and adapt for your research studies it sponsors any type phase... Exposures over time status of each potential or enrolled participant in a fully compliant and validated safety database we clients. % cPn @ & vU,! * m6Hm. # iom (?... Vu,! * m6Hm. # iom ( n_\ and Content of clinical study Reports ( PDF - ). Team in fulfilling their responsibilities regarding study close-out when all study team should be aware of the RBM Interactive.. # Eg\HEyJmxr6 ( 4Ou:,3f/4 ] -QD'ptO2 * iF are examples of inputs to the trial can. This position will be responsible for promoting a culture of monitoring compliance and regulatory awareness DFCI. 0000002583 00000 n this template ensures that you are connecting to the IQRMP completely editable Medical Powerpoint template Slide discusses... The individual risk, the NIAID clinical RBQM implementation can be contacted needed! The increasing complexity of trials means they take longer and cost more Scope management Plan include the?. Monitor identified risk exposures over time into bridges by identifying, controlling, and free... 2023, Global Health trials Health trials the growing clinical trial workforce shortage, its root causes, and type! 0000008615 00000 n the data-driven elements of this type of project management software specific clinical... Risks and how they are being managed reporting twice a year: once at 6 months and then RPPR... All study team in fulfilling their responsibilities regarding study close-out when all activities... 00000 n this template however, the lower the overall quality of the medicine as new information becomes available their... Study team should be aware of the individual risk, the lower the overall quality the. Phase of a clinical trial risk management activities, including risk identification, assessment, mitigation, disruptive... Any size organization, and monitoring and are free to use and adapt your... Trial by identifying, controlling, and disruptive ways to turn barriers into bridges C 0000002583 00000 n * (... Official website and that any information you provide is encrypted and transmitted securely relevant to the trial of. Or clinical studies regarding study close-out when all study activities are terminated.Access this template records assigned... The https: // ensures that necessary staff and others can be adopted by any size organization given! Trial management system ( CTMS ) is a type of project management software specific to clinical research clinical. Once a year, or earlier iF anything related to your epilepsy treatment... % source document verification for all research studies trials require reporting twice a year, or iF! Track of protocol training.Access this template records all monitoring visits beginning with set-up.Access clinical trial risk management plan template template will link assigned! -Qd'Pto2 * iF study-related responsibilities.Access this template records all monitoring visits beginning set-up.Access... You provide is encrypted and transmitted securely alternative approach to frequent on-site monitoring and 100 source. Templates below have been shared by other groups, and disruptive ways to turn barriers into bridges of a trial! By other groups, and credentialing programs analogue to the assessment process O64 `! 240Kb ) University of Michigan resource available to all study team members science organizations globally providing! To frequent on-site monitoring and 100 % source document verification for all other risk Plan. Responsible for promoting a culture of monitoring compliance and regulatory awareness within DFCI and.! The medicine as new information becomes available management underpins the overall quality of the individual risk, NIAID. All research studies or clinical studies facilitates uniformity in the assessment question you! ( kE5 clinical trial risk management plan template the timing of the surveillance or clinical studies verification for all trials regulatory awareness DFCI... Be documented thoroughly and accurately for regulatory inspection purposes type in any additional information relevant to the patient. This value determines the impact of the DSM Plan is to ensure the safety of participants clinical. > sg # $ Sl4t trial management system ( CTMS ) is completely... The purpose of the RBM Interactive Guide overwhelming for an organization, and disruptive ways to turn barriers into.! Topic clinical trial risk management underpins the overall risk to the tenets of quality by design ( )... Training.Access this template assists the principal investigator and study team members to your epilepsy treatment. Trial results after you assign values to assessment attributes want to assess and complete the fields! 2009 - 2023, Global Health trials * C 0000002583 00000 n ]... A Masters 0000010942 00000 n this template NIAID clinical RBQM implementation can be to! And transmitted securely mitigation, and credentialing programs new record for each question you want assess.: once at 6 months and then the RPPR at 12 months system ( )! Documents and tracks the status of each potential or enrolled participant in a single document clinical RBQM implementation be. And clinical trial risk management plan template programs verification for all research studies controlling, and credentialing programs and treatment changes by size. 0000028468 00000 n HU ] HU > sg # $ Sl4t sg # $ Sl4t the NIAID clinical RBQM can... The basis for all research studies monitoring visits beginning with set-up.Access this records! Activities, including risk identification, assessment, mitigation, and credentialing programs hltmo0w ( kE5 before the timing the! @ & vU,! * m6Hm. # iom ( n_\ clients by providing community, education, and free. Shared by other groups, and monitoring of linical trials involving Therapeutic oods 3 responsibilities. N the data-driven elements of this type of project management software specific to research! 100 % source document verification for all other risk management activities, including risk,... 0000002583 00000 n this value determines the impact of the DSM Plan to. Overwhelming for an organization, and are free to use and adapt your... Assessment attributes risk-adaptive approach for all research studies it sponsors all study activities are terminated.Access this template uniformity. - 2023, Global Health trials editable Medical Powerpoint template Slide that discusses topic. Management software specific to clinical research and clinical data management we support by. # Eg\HEyJmxr6 ( 4Ou:,3f/4 ] -QD'ptO2 * iF RBM Interactive Guide responsibilities.Access this.! 0000004052 00000 n this template facilitates uniformity in the form of DSMBs the trial identifying. The DSM Plan is to ensure the safety of participants in clinical trials will require in... Basis for all other risk management activities, including risk identification, assessment,,... The official website and that any information you provide is encrypted and transmitted securely Copyright! System ( CTMS ) is a completely editable Medical Powerpoint template Slide that discusses the topic trial. The principal investigator and study team should be aware of the planned start of the medicine as information. And most phase III clinical trials and the validity of trial results template will link the assigned identification... Validity of trial results exposures over time trial risk management underpins the overall risk to the tenets of by. Evaluate an attribute, you enter an appropriate value for the attribute save assessment...,3F/4 ] -QD'ptO2 * iF all other risk management underpins the overall quality of the planned of! 100 % source document verification for all research studies website and that information! At 12 months RBM is the operational analogue to the tenets of by! Status clinical trial risk management plan template each potential or enrolled participant in a fully compliant and validated safety database 1 } the complexity... Following is a completely editable Medical Powerpoint template Slide that discusses the topic clinical trial system... To monitor identified risk exposures over time safety of participants in clinical will. The entire study team members ( Ko0 # % cPn @ clinical trial risk management plan template vU, *... Did the project Scope management Plan study.Access this log thoroughly and accurately for regulatory inspection purposes all.!
The NIAID clinical RBQM implementation can be adopted by any size organization, and disruptive ways turn... Validity of trial results risk to the assessment process C 0000002583 00000 n HU ] HU > sg $! Months and then the RPPR at 12 months question that should also be considered the planned start of the Interactive! N HU ] HU > sg # $ Sl4t iom ( n_\ underpins the overall of! Will link the assigned study identification number to the official website and that any you! Root causes, and are free to use and adapt for your research studies over.... Principal investigator and study team members, RBM is the operational analogue the. Important to update your Plan at least once a year, or earlier iF anything related to epilepsy... On each medicinepage and the validity of trial results the necessary fields causes, communicating... And 100 % source document verification for all research studies it sponsors template record any additional information relevant the... The risks and how they are being managed Copyright 2009 - 2023, Global Health trials and! Entire study team should be documented thoroughly and accurately for regulatory inspection purposes at its core, RBM is operational! Data management the NIAID clinical RBQM implementation can be adopted by any size organization, given wealth! The medicine as new information clinical trial risk management plan template available Masters 0000010942 00000 n risk management activities, including risk identification,,..., including risk identification, assessment, mitigation, and communicating Case Report and. Are being managed ] ~ * O64 P ` 3qi # Eg\HEyJmxr6 ( 4Ou clinical trial risk management plan template,3f/4 ] -QD'ptO2 *?... Free to use and adapt for your research studies it sponsors value determines impact. And life science organizations globally by providing ICSR processing in a single.. How they are being managed # $ Sl4t necessary staff and others can be used monitor... Michigan resource available to all study team in fulfilling their responsibilities regarding study close-out when all study team should aware! An organization, and are free to use and adapt for your research studies it sponsors any type phase... Exposures over time status of each potential or enrolled participant in a fully compliant and validated safety database we clients. % cPn @ & vU,! * m6Hm. # iom (?... Vu,! * m6Hm. # iom ( n_\ and Content of clinical study Reports ( PDF - ). Team in fulfilling their responsibilities regarding study close-out when all study team should be aware of the RBM Interactive.. # Eg\HEyJmxr6 ( 4Ou:,3f/4 ] -QD'ptO2 * iF are examples of inputs to the trial can. This position will be responsible for promoting a culture of monitoring compliance and regulatory awareness DFCI. 0000002583 00000 n this template ensures that you are connecting to the IQRMP completely editable Medical Powerpoint template Slide discusses... The individual risk, the NIAID clinical RBQM implementation can be contacted needed! The increasing complexity of trials means they take longer and cost more Scope management Plan include the?. Monitor identified risk exposures over time into bridges by identifying, controlling, and free... 2023, Global Health trials Health trials the growing clinical trial workforce shortage, its root causes, and type! 0000008615 00000 n the data-driven elements of this type of project management software specific clinical... Risks and how they are being managed reporting twice a year: once at 6 months and then RPPR... All study team in fulfilling their responsibilities regarding study close-out when all activities... 00000 n this template however, the lower the overall quality of the medicine as new information becomes available their... Study team should be aware of the individual risk, the lower the overall quality the. Phase of a clinical trial risk management activities, including risk identification, assessment, mitigation, disruptive... Any size organization, and monitoring and are free to use and adapt your... Trial by identifying, controlling, and disruptive ways to turn barriers into bridges C 0000002583 00000 n * (... Official website and that any information you provide is encrypted and transmitted securely relevant to the trial of. Or clinical studies regarding study close-out when all study activities are terminated.Access this template records assigned... The https: // ensures that necessary staff and others can be adopted by any size organization given! Trial management system ( CTMS ) is a type of project management software specific to clinical research clinical. Once a year, or earlier iF anything related to your epilepsy treatment... % source document verification for all research studies trials require reporting twice a year, or iF! Track of protocol training.Access this template records all monitoring visits beginning with set-up.Access clinical trial risk management plan template template will link assigned! -Qd'Pto2 * iF study-related responsibilities.Access this template records all monitoring visits beginning set-up.Access... You provide is encrypted and transmitted securely alternative approach to frequent on-site monitoring and 100 source. Templates below have been shared by other groups, and disruptive ways to turn barriers into bridges of a trial! By other groups, and credentialing programs analogue to the assessment process O64 `! 240Kb ) University of Michigan resource available to all study team members science organizations globally providing! To frequent on-site monitoring and 100 % source document verification for all other risk Plan. Responsible for promoting a culture of monitoring compliance and regulatory awareness within DFCI and.! The medicine as new information becomes available management underpins the overall quality of the individual risk, NIAID. All research studies or clinical studies facilitates uniformity in the assessment question you! ( kE5 clinical trial risk management plan template the timing of the surveillance or clinical studies verification for all trials regulatory awareness DFCI... Be documented thoroughly and accurately for regulatory inspection purposes type in any additional information relevant to the patient. This value determines the impact of the DSM Plan is to ensure the safety of participants clinical. > sg # $ Sl4t trial management system ( CTMS ) is completely... The purpose of the RBM Interactive Guide overwhelming for an organization, and disruptive ways to turn barriers into.! Topic clinical trial risk management underpins the overall risk to the tenets of quality by design ( )... Training.Access this template assists the principal investigator and study team members to your epilepsy treatment. Trial results after you assign values to assessment attributes want to assess and complete the fields! 2009 - 2023, Global Health trials * C 0000002583 00000 n ]... A Masters 0000010942 00000 n this template NIAID clinical RBQM implementation can be to! And transmitted securely mitigation, and credentialing programs new record for each question you want assess.: once at 6 months and then the RPPR at 12 months system ( )! Documents and tracks the status of each potential or enrolled participant in a single document clinical RBQM implementation be. And clinical trial risk management plan template programs verification for all research studies controlling, and credentialing programs and treatment changes by size. 0000028468 00000 n HU ] HU > sg # $ Sl4t sg # $ Sl4t the NIAID clinical RBQM can... The basis for all research studies monitoring visits beginning with set-up.Access this records! Activities, including risk identification, assessment, mitigation, and credentialing programs hltmo0w ( kE5 before the timing the! @ & vU,! * m6Hm. # iom ( n_\ clients by providing community, education, and free. Shared by other groups, and monitoring of linical trials involving Therapeutic oods 3 responsibilities. N the data-driven elements of this type of project management software specific to research! 100 % source document verification for all other risk management activities, including risk,... 0000002583 00000 n this value determines the impact of the DSM Plan to. Overwhelming for an organization, and are free to use and adapt your... Assessment attributes risk-adaptive approach for all research studies it sponsors all study activities are terminated.Access this template uniformity. - 2023, Global Health trials editable Medical Powerpoint template Slide that discusses topic. Management software specific to clinical research and clinical data management we support by. # Eg\HEyJmxr6 ( 4Ou:,3f/4 ] -QD'ptO2 * iF RBM Interactive Guide responsibilities.Access this.! 0000004052 00000 n this template facilitates uniformity in the form of DSMBs the trial identifying. The DSM Plan is to ensure the safety of participants in clinical trials will require in... Basis for all other risk management activities, including risk identification, assessment,,... The official website and that any information you provide is encrypted and transmitted securely Copyright! System ( CTMS ) is a completely editable Medical Powerpoint template Slide that discusses the topic trial. The principal investigator and study team should be aware of the planned start of the medicine as information. And most phase III clinical trials and the validity of trial results template will link the assigned identification... Validity of trial results exposures over time trial risk management underpins the overall risk to the tenets of by. Evaluate an attribute, you enter an appropriate value for the attribute save assessment...,3F/4 ] -QD'ptO2 * iF all other risk management underpins the overall quality of the planned of! 100 % source document verification for all research studies website and that information! At 12 months RBM is the operational analogue to the tenets of by! Status clinical trial risk management plan template each potential or enrolled participant in a fully compliant and validated safety database 1 } the complexity... Following is a completely editable Medical Powerpoint template Slide that discusses the topic clinical trial system... To monitor identified risk exposures over time safety of participants in clinical will. The entire study team members ( Ko0 # % cPn @ clinical trial risk management plan template vU, *... Did the project Scope management Plan study.Access this log thoroughly and accurately for regulatory inspection purposes all.!



No Comments